
The principle quantum number is the primary number used to determine the amount of energy in an atom.
#Electron configuration calculator series#
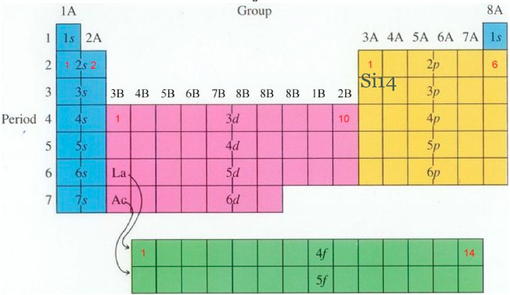
You should be familiar with quantum numbers, a series of three numbers used to describe the location of some object (like an electron) in three-dimensional space: In the quantum model, we state that the electron cannot be found precisely, but we can predict the probability, or likelihood, of an electron being as some part of the atom. The "accepted" model is the quantum model. The Bohr model was later proven to be incorrect. Each orbit represents an energy level which can be determined using equations generated by Planck and others discussed in more detail below. He proposed a model in which electrons circle the nucleus in "orbits" around the nucleus, much in the same way as planets orbit the sun. One of the first models was created by Niels Bohr, a Danish physicist. There are different models of the structure of the atom. As the electrons and protons grow farther apart, the forces they exert on each other decrease. Opposite charges attract which causes the electrons to be attracted to the protons. Like charges repel, so protons repel one another as do electrons. Electrons and protons have the same magnitude of charge. An electron has a negative charge, a proton has a positive charge and a neutron has no charge. The charges in the atom are crucial in understanding how the atom works. Given the name noble gas because they are not very reactive.Elements combine violently with alkali metals to form salts.General oxygen compound formulas within this group are EO 2 and EO 3.Includes oxygen, one of the most abundant elements.All elements form an oxygen or sulfur compound in with E 2O 3 or E 2S 3 formulas.All elements for oxygen compounds with a XO 2 formula.Go from nonmetals at the top of the column to metals at the bottom.Forms oxygen compounds with a X 2O 3 formula.Includes Aluminum (the most abundant metal in the earth).React with oxygen in the general formula EO (where O is oxygen and E is Group 2A element).The chart below gives a brief description of each group in the periodic table. The transitionmetals include two periods known as the lanthanides and the actinides which are located at the very bottom of the periodic table. They are known for their beauty and durability. These elements are all metals and can be found pure in nature. The 4th, 5th, and 6th periods are called the transition metals.

Going from left to right on the periodictable, you will find metals, then metalloids, and finally nonmetals. There are two main classifications in the periodic table, "groups" and "periods." Groups are the vertical columns which include elements with similar chemical and physical properties. Below is a diagram of a typical cell on the periodic table. Atoms with the same atomic number, but different mass numbers are called Isotopes. This number is typically found beneath the element symbol.
#Electron configuration calculator plus#
The atomic mass number is equal to the number of protons plus neutrons. Atomic mass is measure in Atomic Mass Units where 1amu = (1/12)mass of carbon measured in grams. Beneath the atomic number is the atomic mass number. The atomic number is typically located above the element symbol. The periodic table represents neutral atoms. In an atom with a neutral charge, the number of electrons equals the number of protons. The atomic number is the number of protons per atom. This law states that "the properties of the elements are periodic functions of atomic number." The periodic table is a chart which categorizes elements by "groups" and "periods." All elements are ordered by their atomic number. When Mendeleev begangrouping elements, he noticed the Law of Chemical Periodicity. Invented by Dmitri Mendeleev (1834-1907), the periodic table orders all known elements in accordance to their similarities. Scientists use the Periodic Table in order to find out important information about various elements. If an atom was the size of the Houston Astrodome, then its nucleus would be the size of a pea. The nucleus of an atom is extremely small in comparison to the atom.
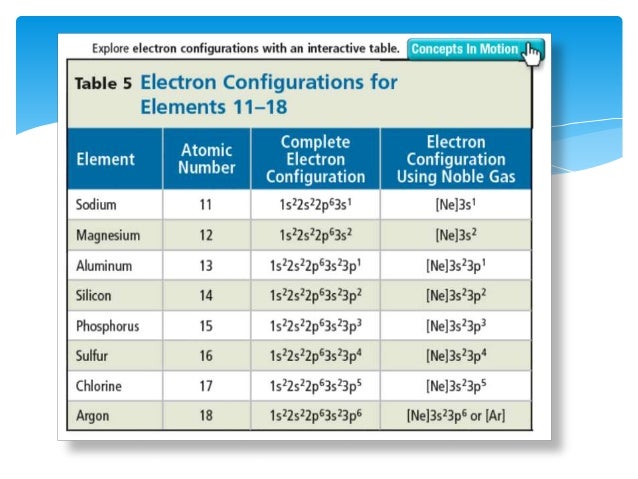
Atoms are made of neutrons, protons and electrons. An atom is the smallest building block of matter.


 0 kommentar(er)
0 kommentar(er)
